Transposition of the Great Arteries (TGA)
Editor's note: Madeleine Howell-Moroney created the above image for this article. You can find the full image and all relevant information here.
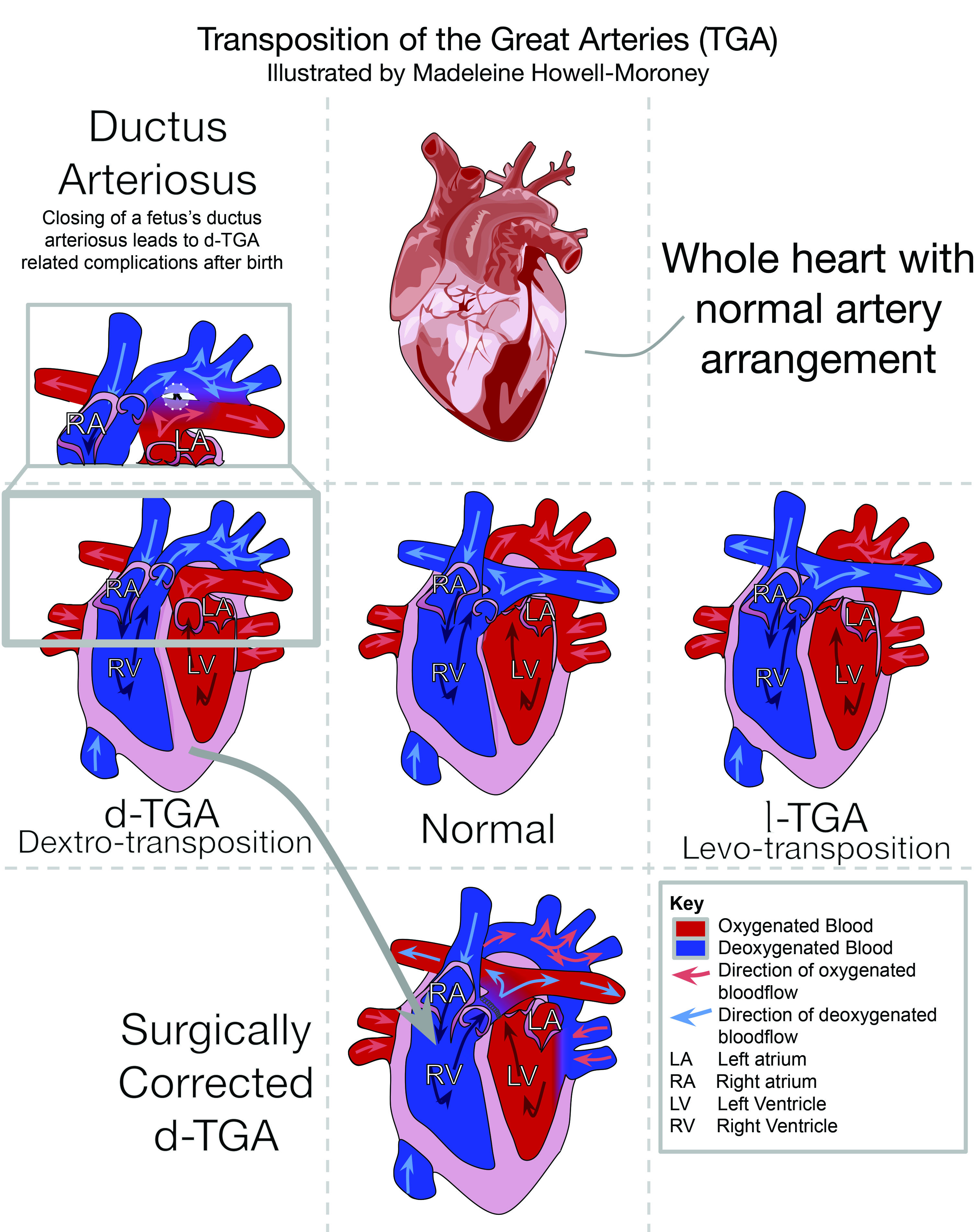
Transposition of the great arteries or TGA is a potentially fatal congenital heart malformation where the pulmonary artery and the aorta are switched. The switch means that the aorta, which normally carries oxygenated blood, carries deoxygenated blood. There are two types of the malformation, d-TGA where no oxygen reaches the body and l-TGA where some oxygenated blood circulates. In the US, the Centers for Disease Control estimate that about 1,901 infants are born each year with TGA, or about one for every 2,000 births. Throughout history, physicians classified TGA as a condition that causes blue babies and hypothesized it was a fatal condition. With the development of corrective surgeries, studies on the causes of TGA, and improved prenatal diagnosis have allowed for the survival rate for those with TGA to approach almost one hundred percent.
The heart is has a systemic circuit and a pulmonary circuit, each comprised of two chambers, an atrium on top and a ventricle beneath that. The systemic circuit is located on the left side of the heart, and pumps blood oxygenated in the lungs out from the heart to the rest of the body through the aorta. The pulmonary circuit is located on the right side of the heart, and takes the deoxygenated blood returning to the lungs for oxidation through the pulmonary artery.
Many fetuses with TGA survive to full-term but die soon after birth when the ductus arteriosus closes. The ductus arteriosus is a hole between the pulmonary artery and the aorta in the fetus, which allows for blood to mix in utero. The ductus arteriosus closes at birth so that oxygenated and deoxygenated blood no longer mix, because the lungs inflate to oxygenate blood.
After the ductus arteriosus closes, the survival of an infant can depend greatly on the type of TGA they have. The d-TGA form is more severe because the arteries connect to the wrong sides of the heart, leading to cyanosis. Instead of blood leaving the pulmonary system and going to the aorta, the blood immediately returns to the lungs. Blood from the body is left circling the systemic circuit with no oxygen. Infants with d-TGA risk organ failure due to their organs not receiving the oxygenated blood and nutrients they need to function, making it the more severe form of TGA. In l-TGA, the organs get oxygenated blood because the right and left ventricles are also switched, which means that deoxygenated blood still gets to the lungs. That form of TGA has fewer side effects because the body receives oxygenated blood and the lungs get deoxygenated blood. Typically that type of TGA does not require surgical correction.
Matthew Baillie described the anatomical structure of TGA in 1793 while at St George’s Hospital in London, England. Baillie described an affected infant while still alive and later reported findings of the infant’s autopsy in his book The Morbid Anatomy of some of the Most Important Parts of the Human Body. Baillie described the living child as having unusually bright coloration, with colder than normal skin, and reported that the infant breathed normally. After the autopsy, Baillie described the anatomy of TGA and found that the aorta and pulmonary artery arose from the wrong ventricles. He concluded that the coloration of the skin was caused by the flowing of blood in two distinct circuits, never mixing except through the limited amount that flowed through the mostly closed ductus arteriosus, which was so small that he was only able to fit a crow quill through. Baillie’s clinical description of TGA allowed other physicians to begin characterizing the condition.
After Baillie described the anatomy, John Richard Farre, a physician in London, England, named the defect a transposition of the aorta and pulmonary artery in his 1814 book Pathological Researches on Malformations of the Human Heart. Farre mentioned not only the term transposition, but also listed symptoms of several cases, including the first one Baillie had mentioned. He described the blue coloration of those affected with cyanotic heart malformations, including but not limited to TGA. Farre called those infants blue babies. That grouping of blue babies included TGA and tetralogy of fallot, a similar heart defect where the aorta is misplaced, a condition that slows blood flow to the lungs. Farre also noted that the infants born with that condition had irregular respiration, poor nutrition, neurological side effects such as seizures, loss of consciousness, paralysis, and intermittent quickening of the heart’s pace. However, few surgical corrections existed for those with TGA until the early-1900s, when surgical corrections began.
In 1939 Robert Gross and John Hubbard, who worked as physicians in Boston, Massachusetts, performed the first correction of a congenital heart condition where the ductus arteriosus fails to close after birth. They performed surgery on a seven-and-a-half-year-old girl and permanently closed her ductus arteriosus with a silk tie, which allowed Gross and Hubbard to cut off the ductus arteriosus. Gross and Hubbard published their results in 1939 article titled “Surgical Ligation of a Patent Ductus Arteriosus.” Helen Taussig cited that corrective surgery, as inspiration for her first steps toward exploring surgical correction for blue babies at Johns Hopkins University, in Baltimore, Maryland, in 1944. Taussig along with Alfred Blalock, a surgeon, and his assistant Vivien Thomas, developed the Blalock-Taussig shunt to divert blood from the aorta to receive oxygenated blood for those with tetralogy of fallot. That operation led Blalock to develop surgical corrections for TGA, specifically for d-TGA.
In 1950, Blalock along with C. Rollins Hanlon developed a surgery to artificially create a hole between the atria of the heart known as atrial septal defect, to allow for mixing of oxygenated and deoxygenated blood. As of 1953, the mortality for those with TGA was eighty-six percent within six months of delivery. The atrial septal defect was created and tested on an eight-day-old infant who had low body temperature, rapid breathing, and swelling of the feet and eyelids. Surgeons created a hole between the atria, blood was allowed to mix, and the infant survived as of six months post operation.
In 1953, Thomas Baffes, who was a physician in Chicago, Illinois, began to develop an operation to help relieve the effects of TGA. Baffes attempted several times and operated on the first human, but the operation yielded few results. On 6 May 1955, Baffes performed his first successful surgery. That surgery was done with no cardiopulmonary bypass system, which is a system that circulates oxygen within a patient’s body, and the patient’s body temperature was lowered to thirty degrees Celsius. After this first success, Baffes went on to continue operating on children with TGA. Between 1955 and 1956, Baffes performed thirty-six operations, of which eighteen died, leaving a fifty percent mortality rate. In 1959 the next surgical correction arose called the Senning Procedure, developed by Åke Senning.
Senning followed the routine of rerouting blood through the atria in several steps, creating an atrial septal defect, manipulating the heart by surgeons to reroute blood, and recreating of the atria. Surgeons rerouted blood from the vena cava and a vessel on the heart venous systems to the left atrium, instead of the right atrium, where they initially drained. That allows blood to flow from the venous system, which carries deoxygenated blood in those with TGA, into the left atrium, where blood is carried to the pulmonary artery for oxygenation, and the right atrium pumps oxygenated blood to the body instead of the left atrium. Then surgeons recreate the atria for an arterial and venous atrium through suturing, folding, and incisions. While that surgery was successful and corrected the condition of TGA, it was a difficult surgery to perform and was replaced by a different method of surgical correction.
In 1963, William Mustard, who worked at the Hospital for Sick Children in Toronto, Ontario, developed a corrective surgery that became the primary corrective surgery for TGA until 1998. Mustard first operated on Maria Surnoski, a ward of the state awaiting adoption, when she was dying. Mustard performed his experimental operation in hopes of saving her, and was successful. In 1968, Mustard published his surgical technique to fix TGA in an article called “The Role of Surgery in the Treatment of Transposition of the Great Vessels.” His method resulted in a reversal of typical blood flow, but still allowed for the blood to be oxygenated and travel throughout the body. That surgery lasted until 1998 when it was replaced with the arterial switch operation.
The arterial switch operation is the most common operation to correct TGA as of 2016. Adib Jatene developed the arterial switch operation in 1975 in São Paulo, Brazil. The operation was the first to realign the vessels’ incorrect anatomical position. The ability for surgeons to perform open-heart surgery allowed them to correct the transposition. First, surgeons isolate the pulmonary artery, the aorta, and the coronary arteries of the heart. They clamp the infant’s vessels and cut the coronary arteries and place them on the pulmonary artery to ensure oxygenated blood gets to the heart. Then surgeons relocate the pulmonary artery and the aorta to their proper anatomical position and suture the cut on both the aorta and pulmonary artery. Shortly after the development of the atrial switch operations, scientists began studies to understand the causes of TGA.
From 1981 to 1989, US physicians Charlotte Ferencz, Judith Ruben, Christopher Loffredo, and Carol Magee studied potential causes for congenital heart diseases, including TGA. The study clarified the role of harmful chemicals that cause birth defects, called teratogens. The study resulted in a link between maternal exposure to herbicides or rodenticides and development of TGA. They found a 7.8 percent occurrence of TGA when the fetus was exposed to herbicides and rodenticides. Of sixty-six children with TGA, 2.8 percent reported maternal exposure to herbicides, and 4.7 percent reported maternal exposure to rodenticides in the first trimester of pregnancy. Other teratogens were explored during this time frame as well, including retinoic acid, which was explored as early as 1961.
Exposure to retinoic acid is one of the most well understood risk factors in the development of TGA. Since 1961, scientists observed that a high dose of vitamin A correlated to an increase in TGA. Harold Karter and Josef Warkanny performed experiments on mice treated with retinoic acid, which displayed TGA at an eighty to ninety percent rate. More current research includes the 1995 and 1997 research by Hiroshi Yasui, in Tokyo, Japan, on retinoic acid in mice. Yasui and his team built on the research of Karter and Warkanny, and examined how retinoic acid impacted embryological development.
Another possibility for increased risk of TGA is maternal influence such as diabetes. A study done throughout 2000 and 2001 at the King Khalid University Hospital in Riyadh, Saudi Arabia, involved 100 births with diabetic mothers and showed an increased risk for congenital heart defects in the diabetic population, with a one percent incidence of TGA in the pregnant women studied with insulin-dependent diabetes. Abu-Sulaiman stated that the results demonstrated a need for prenatal screening in this population.
Advanced maternal age is another maternal risk factor that puts the fetus at risk for developmental issues such as TGA. Assia Miller, a medical doctor, with her partners, published an article in the American Journal of Medical Genetics that showed a link between advanced maternal age and the incidence of TGA through a study of five counties in Atlanta, Georgia. The study observed 5,289 infants, as well as fetuses’ from 1968 to 2005, who had an isolated congenital heart defect. Miller observed that the adjusted prevalence ratio for mothers who had infants or fetuses with TGA increased with maternal age. Pregnant women who were over thirty-five years old were at a higher risk than younger pregnant women.
Damien Bonnet and his team in Paris, France recommend diagnosing TGA on fetuses versus after delivery. They studied the benefits of diagnosing before and after birth, and found that diagnosing before delivery was better overall than diagnosing after delivery. Bonnet and his team found those diagnosed before delivery were admitted to the ICU unit between 1.4 to 2.9 hours, while those diagnosed after delivery weren’t admitted until forty-six to one hundred hours after delivery. They also found an increased risk for complications including multi-organ failure, neurological distress, and acidic blood that can lead to shock or death in infants diagnosed after birth.
Sources
- Abu-Sulaiman, Riyadh M., B. Subaih “Congenital Heart Disease in Infants of Diabetic Mothers: Echocardiographic Study” Pediatric Cardiology 25 (2004): 137–40.
- Aird, W.C. “Discovery of the cardiovascular system: from Galen to William Harvey.” Journal of Thrombosis and Haemostasis 9 (2011): 118–29. http://onlinelibrary.wiley.com/doi/10.1111/j.1538-7836.2011.04312.x/pdf (Accessed February, 2015).
- Baffes, Thomas “Willis J. Potts: His Contributions to Cardiovascular Surgery” The Annals of Thoracic Surgery 44 (1987):92–6. http://www.annalsthoracicsurgery.org/article/S0003-4975%2810%2962371-5/abstract (Accessed March 2015).
- Baillie, Matthew. The Morbid Anatomy of Some of the Most Important Parts of the Human Body. London: Printed for J. Johnson and G. Nicol, 1793. https://archive.org/details/morbs00bail (Accessed May 3, 2016).
- Blalock, Alfred, and Helen B. Taussig. "The Surgical Treatment of Malformations of the Heart: in Which There is Pulmonary Stenosis or Pulmonary Atresia." Journal of the American Medical Association 128 (1945): 189–202.
- Bonnet, Damien, Anna Coltri, Gianfranco Butera, Laurent Fermont, Jérôme Le Bidois, Jean Kachaner, and Daniel Sidi. “Detection of Transposition of the Great Arteries in Fetuses Reduces Neonatal Morbidity and Mortality” Circulation 99 (1999): 916–8. http://www.cdc.gov/ncbddd/heartdefects/tga.html (Accessed May 3, 2016).
- CDC “Facts about Transposition of the Great Arteries” http://www.cdc.gov/ncbddd/heartdefects/tga.html (Accessed March 2015)
- Constantine, Mavroudis ; Backer, Carl ; Siegel, Allison ; Gevitz, Melanie “Revisiting the Baffes Operation: Its Role in Transposition of the Great Arteries” Annals of Thoracic Surgery (2014):373–7. http://www.annalsthoracicsurgery.org/article/S0003-4975(13)02246-7/fulltext (Accessed February 2015)
- Dibardino, Daniel, Allison, Andrew ; Vaughn, William ; McKenzie, Dean ; Fraser, Charles “Current Expectations for Newborns Undergoing the Arterial Switch Operation” Annals of Surgery 239 (2004): 588–96. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1356266/ (Accessed February, 2015)
- Dunlop, Marilyn and Richard Kerr. Bill Mustard: Surgical Pioneer. Toronto: Dundurn, 1989. http://php.ams-inc.on.ca/files/b_mustard.pdf (Accessed September 5, 2014)
- Farre, John Richard. Pathological Researches on Malformations of the Human Heart. London: Longman, Hurst, Rees, Orme, and Brown, Paternoster-Row, 1814. https://archive.org/stream/pathologicalrese00farr#page/n9/mode/2up (Accessed February, 2015)
- Ferencz, Charlotte, Judith Ruben, Christopher Loffredo, and Carol Magee. “Perspectives in Pediatric Cardiology: The Baltimore-Washington Infant Study 1981-1989.” Cardiology in the Young 4 (1993): 33–62.
- Fowler, R.S., William Mustard, and George Trusler. "The Role of Surgery in the Treatment of Transposition of the Great Vessels." Canadian Medical Association 91 (1968): 1096–100. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1928362/ (Accessed March 12, 2016).
- Gotsman, Mervyn. “Creation of an Atrial Septal Defect in Transposition of the Great Vessels.” Thorax (1965): 574–8. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1018987/?page=1 (Accessed March 2015)
- Gross, Robert and Hubbard, John “Surgical Ligation of a Patent Ductus Arteriosus.” Journal of the American Medical Association 112 (1939): 729–731.
- Kalter, Harold and Josef Warkany “Experimental Production of Congenital Malformations in Strains of Inbred Mice by Maternal Treatment with Hypervitaminosis A.” The American Journal of Pathology 38 (1961) 1–21. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1942316/ (Accessed May 3, 2016).
- Kam, Richard Kin Ting, Deng Yi, Yonglong Chen, Hui Zhao. “Retinoic Acid Synthesis and Functions in Early Embryonic Development” Cell and Bioscience 2 (2012): 1. http://www.cellandbioscience.com/content/2/1/11 (Accessed October 2014)
- Konstantinov, Igor, Vladimir Alexi-Meskishivilli, William Williams, Robert Freedom, and Richard Van Praagh “Atrial Switch Operation: Past, Present and Future.” The Annals of Thoracic Surgery 77 (2004) 2250–8. http://www.annalsthoracicsurgery.org/article/S0003-4975(03)02050-2/fulltext (Accessed May 3, 2016).
- Loffredo, Christopher, David Wilson, Charlotte Ferencz. “Maternal Diabetes: an Independent Risk Factor for Major Cardiovascular Malformations with Increased Mortality of Affected Infants.” Teratology 64 (2001): 98–106. http://www.ncbi.nlm.nih.gov/pubmed/11460261 (Accessed May 3, 2016).
- Martins,Paula and Eduardo Castela. “Transposition of the Great Vessels” OrphanNet Journal of Rare Diseases 3 (2008) http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2577629/ (Accessed May 3, 2016).
- Miller, Assia, Tiffany Riehle‐Colarusso, Csaba Siffel, Jaime L. Frías, and Adolfo Correa. “Maternal Age and Prevalence of Isolated Congenital Heart Defects in an Urban Area of the United States” American Journal of Medical Genetics 9 (2011): 2137–45. http://www.ncbi.nlm.nih.gov/pubmed/21815253 (Accessed October 2014).
- Senning, Åke "Surgical Correction of Transposition of the Great Vessels." Surgery 45 (1959): 966–80.
- Unolt, Marta, Carolina Putotto, Lucia M. Silvestri, Dario Marino, Alessia Scarabotti, Valerio Massaccesi, Angela Caiaro, Paolo Versacci, and Bruno Marino. “Transposition of the Great Arteries: New Insights into the Pathogenesis” Frontiers in Pediatrics 1 (2013) http://www.ncbi.nlm.nih.gov/pubmed/24400257 (Accessed October 30, 2014).
- Yasui, Hiroshi, Makoto Nakazawa, Masae Morishima, Masahiko Ando, Atsuyoshi Takao, and Eizo Aikawa. “Cardiac Outflow Tract Septation Process in the Mouse Model of Transposition of the Great Arteries” Teratology 55 (1997): 353–63. http://www.ncbi.nlm.nih.gov/pubmed/9294880 (Accessed November 10, 2014)
- Yasui, Hiroshi, Makoto Nakazawa, Masae Morishima, Sachiko Miyagawa-Tomita, and Kazuo Momma “Morphological Observations on the Pathogenetic Process of Transposition of the Great Arteries Induced by Retinoic Acid in Mice” Circulation 91 (1995): 2478–86. http://www.ncbi.nlm.nih.gov/pubmed/7729035 (Accessed November 10, 2014)
Keywords
Editor
How to cite
Publisher
Handle
Rights
Articles Rights and Graphics
Copyright Arizona Board of Regents Licensed as Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported (CC BY-NC-SA 3.0)