Human Papillomavirus (HPV) Strains 16 and 18
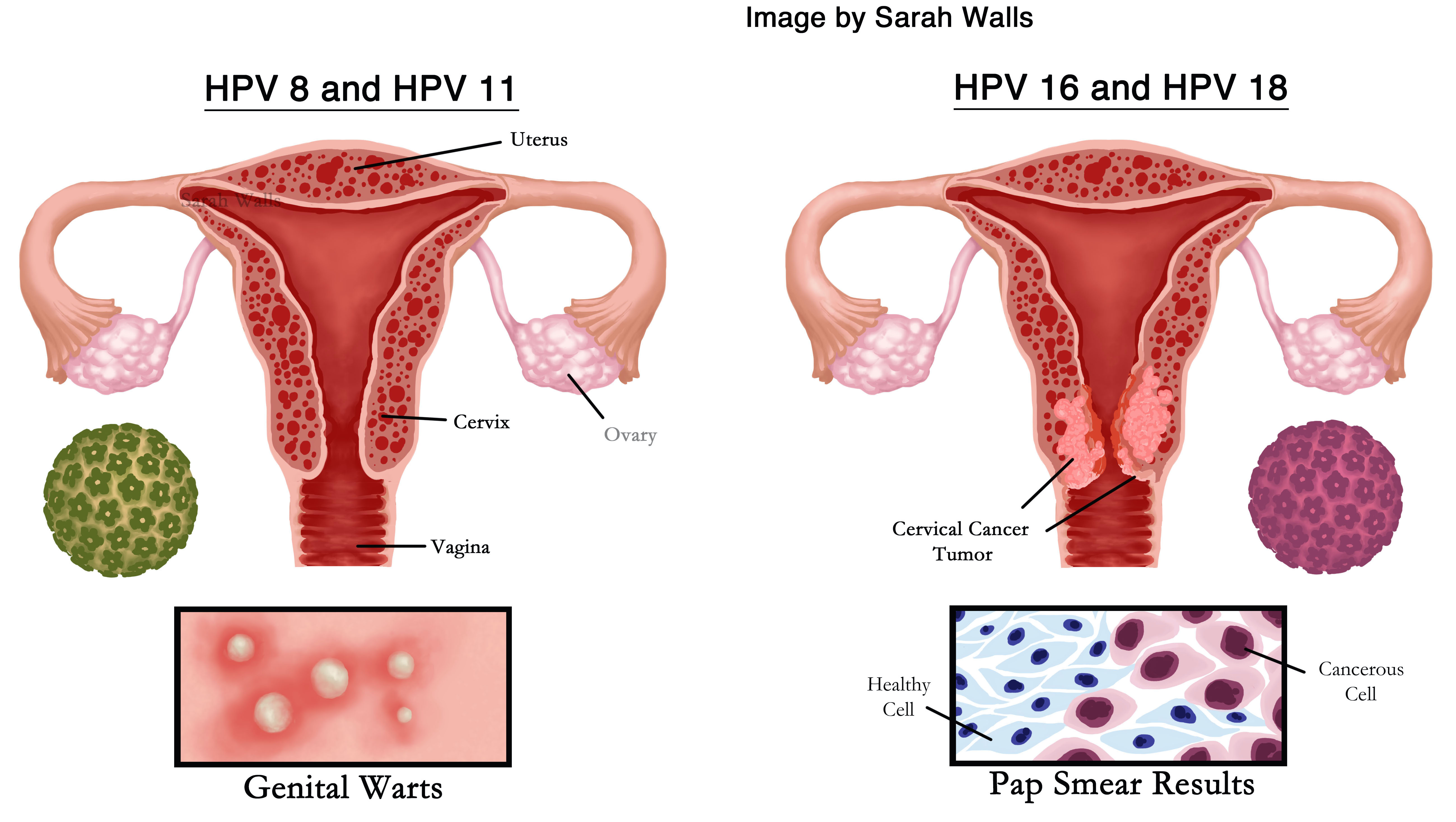
Editor's note: Sarah Walls created the above image for this article. You can find the full image and all relevant information here.
The Human Papillomavirus (HPV) strains 16 and 18 are the two most common HPV strains that lead to cases of genital cancer. HPV is the most commonly sexually transmitted disease, resulting in more than fourteen million cases per year in the United States alone. When left untreated, HPV leads to high risks of cervical, vaginal, vulvar, anal, and penile cancers. In 1983 and 1984 in Germany, physician Harald zur Hausen found that two HPV strains, HPV-16 and HPV-18, caused cervical cancer in women. In the early twenty first century, pharmaceutical companies Merck & Co. and GlaxoSmithKline created HPV vaccines protecting against HPV-16 and HPV-18, which have reduced the number of HPV infections by fifty-six percent in the US. Discovering HPV strains 16 and 18 allowed physicians to test for those cancer-causing cell populations using Pap smears, a diagnostic tool that collects cells from the woman's cervix to identify cancerous cases of HPV infection. By identifying the cancerous strains of HPV-16 and HPV-18 and utilizing preventative measures such as the Pap smear and HPV vaccines, the rates of cervical cancer and other HPV-related cancers have reduced.
HPV and cervical cancer research started with the study of genital warts caused by sexually transmitted viruses with the idea that genital warts eventually led to genital cancers, primarily cervical cancer. In 1928 in the US, physician George Papanikolaou developed early versions of the Pap smear test, a screening test that collects and analyzes cells scraped from the woman's cervix, as a diagnostic test primarily for cervical cancer. Pap smear samples showed one of the first observations of cancer in cervical cells. Harald zur Hausen started his research on HPV after reviewing medical reports that discussed cases of HPV genital warts that progressed to cervical cancer in female patients, and in 1976, he published his hypothesis that HPV caused cervical cancer in the article, "Condylomata Acuminata and Human Genital Cancer.
In his experiments, zur Hausen looked for HPV DNA in genital warts and tumor samples. In the samples, he first identified DNA from HPV strains 6 and 11, two common HPV types that cause genital warts. If HPV strains 6 and 11 from genital warts caused cancer, zur Hausen hypothesized that he would find those same strains in cervical tumor samples. Zur Hausen analyzed cervical tumor samples and found a low prevalence of HPV-6 and HPV-11, but noticed other HPV DNA in the cervical tumor samples that he identified as HPV strains 16 and 18. He then looked for the DNA of HPV strains 16 and 18 in both genital warts and genital tumor samples. He discovered very little HPV-16 and HPV-18 DNA in the genital warts samples and a high prevalence of HPV-16 and HPV-18 DNA in the genital tumor samples. From those results, zur Hausen concluded that HPV-6 and HPV-11 caused genital warts, not cancer, and HPV-16 and HPV-18 caused cervical cancer.
Following zur Hausen's isolation HPV DNA and finding HPV strains 16 and 18 in cervical tumors, scientists began identifying more types of HPV. By identifying more types of HPV and looking for their DNA in tumors, scientists built evidence that some strains of HPV caused cervical cancer and looked for solutions to prevent cervical cancer. Researchers have identified over 150 types of HPV, linking multiple HPV types to HPV-related conditions of warts and cancerous tumors. Scientists have linked other HPV strains, such as HPV-31, HPV-33, and HPV-35, to cervical cancer. Current research correlates HPV infections with an increase in oral cancers, supported by prevalence of HPV-16 DNA in throat cancer tumors. While HPV-16 primarily is known to cause cervical cancer, HPV-16 is also associated with oral cancers due to HPV transmission through oral sex.
HPV is spread sexually through genital skin contact. Subsequent, infection of the epithelial cells, the cells that line the cavities of organs, leads to increased risks of cancer. HPV infection occurs when HPV integrates its own DNA with the DNA in the body's cells. If successful, the HPV DNA is expressed in the body's cells. The cells that express HPV DNA are called permissive cells. Permissive cells enable viral HPV replication, which causes the HPV infection persist in the body. Persistent HPV infections occur when the HPV DNA successfully survives in the body, resulting in long-term chronic infections.
Physicians treat symptoms, such as genital warts caused by HPV-6 and HPV-11, with medication, but medication is not always necessary. HPV infections can eventually go away on its own, though scientists are not entirely sure how. Physicians use Pap smears not only to detect cervical cancer but also HPV-16 and HPV-18 strains that might later lead to cancer. Abnormal Pap smears show abnormal cervical cells, changes primarily caused by HPV-16 and HPV-18. In most cases, abnormal cervical cells revert to normal cells as the HPV infection often resolving on its own. Over time, however, if the cervical cells remain abnormal and if physicians are able to detect the abnormalities early, they remove the cells from the body to prevent the cells from leading to cancer. As of 2016, scientists and physicians have not found treatments for HPV after infection has already occurred and have not found complete explanations as to why HPV infections resolve on their own.
Physicians and scientists advocate for HPV vaccination, a preventative measure to reduce the risk of genital warts and cervical cancer caused by HPV strains 6, 11, 16, and 18. Pharmaceutical companies Merck & Co. and GlaxoSmithKline created two HPV vaccines, Gardasil and Cervarix in the first decade of the twenty-first century. The HPV vaccines act as a preventative method to protect against HPV-related cancers. Gardasil is a vaccine that protects against the HPV strains 6, 11, 16, and 18, which commonly cause both genital warts and cervical cancer. Cervarix is a vaccine that only protects against HPV strains 16 and 18, which primarily cause cervical cancer. Since the HPV vaccine is a preventative measure against HPV, a sexually transmitted disease, the vaccine is intended for young children before they become sexually active to ensure immunity. If the child is vaccinated before he or she becomes sexually active, the child develops immunity by having the antibodies that will recognize and fight off the HPV infection if the child does contract HPV. The HPV vaccine is less effective in sexually active adults because they most likely have already been exposed to HPV.
The Food and Drug Administration (FDA) has approved Gardasil for use in both boys and girls because Gardasil protects against the HPV strains that cause genital warts, symptoms that occur in both men and women. Cervarix, on the other hand, is approved only for use in girls as it only protects against the HPV strains that cause cervical cancer, HPV-16 and HPV-18. Current research demonstrates the effectiveness of the HPV vaccines, showing a significant reduction in cervical cancer prevalence in women. Studies conducted in 2009 and 2012 demonstrated that Gardasil showed a forty-three percent efficacy rate for protecting against cervical cancer and that Cervarix demonstrated a ninety-three percent efficacy rate for protecting against cervical cancer. As of 2016, the Centers for Disease Control and Prevention (CDC) reported that Merck & Co. is working on a new HPV vaccine that protects against nine types of HPV, since more HPV types have been identified and linked to cervical cancer than just HPV-16 and HPV-18.
Following the production of HPV vaccines, some groups disapproved of HPV vaccination. HPV vaccines are sometimes opposed because the vaccines are primarily intended for young children. Some parents who are against vaccinating their children believe that the HPV vaccines may promote promiscuous behavior, are unsafe, or are ineffective. While the US government does not require HPV vaccination, some states have mandated or strongly recommended HPV vaccination as a health precaution for their students. In 2013, the CDC reported that thirty-five percent of adolescent boys and fifty-seven percent of adolescent girls received one or more doses of the HPV vaccine.
Sources
- Apter, D. et. al. "Efficacy of HPV-16/18 AS04-Adjuvanted Vaccine Against Cervical Infection and Precancer in Young Women: Final Event-Driven Analysis of the Randomized, Double-Blind PATRICIA Trial." Clinical and Vaccine Immunology 22 (2015): 361–377. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4375348/ (Accessed May 6, 2016).
- Albrech, Thomas, Boldogh, Isvlan, Fons, Micahel, and Alan S. Rabson. Medical Microbiology . Galveston: University of Texas Medical Branch at Galveston Press, 1996. http://www.ncbi.nlm.nih.gov/books/NBK7979/ (Accessed May 6, 2016).
- Centers for Disease Control and Prevention. "Human Papillomavirus (HPV)." Centers for Disease Control and Prevention. http://www.cdc.gov/hpv/whatishpv.html (Accessed May 6, 2016).
- Centers for Disease Control and Prevention. "HPV Vaccination." Centers for Disease Control and Prevention. http://www.cdc.gov/vaccines/vpd-vac/hpv/ (Accessed May 6, 2016).
- Centers for Disease Control and Prevention. "Human Papillomavirus: Epidemiology and Prevention of Vaccine-Preventable Diseases." Centers for Disease Control and Prevention. http://www.cdc.gov/vaccines/pubs/pinkbook/hpv.html (Accessed May 6, 2016).
- Centers for Disease Control and Prevention. "Human Papillomavirus (HPV) and Oropharyngeal Cancer — Fact Sheet." Centers for Disease Control and Prevention. http://www.cdc.gov/std/hpv/stdfact-hpvandoropharyngealcancer.htm (Accessed May 6, 2016).
- Centers for Disease Control and Prevention. "Making Sense of Your Pap & HPV Test Results." Centers for Disease Control and Prevention. http://www.cdc.gov/std/hpv/pap/ (Accessed May 6, 2016).
- Centers for Disease Control and Prevention. "Treatment." Centers for Disease Control and Prevention. http://www.cdc.gov/hpv/treatment.html (Accessed May 6, 2016).
- Crum, Christopher, and Nuovo, Gerard. Genital Papillomaviruses and Related Neoplasms. New York: 1999.
- Gardasil. "Gardasil." Merck & Co. http://www.gardasil.com/ (Accessed May 6, 2016).
- Gissman, Lutz, Boshart, Michael, Durst, Matthias, Ikenberg, Hans, Wagner, Dieter, and zur Hausen, Harald. "Presence of Human Papillomaviruses in Genital Tumors." Journal of Investigative Dermatology 83 (1984): 26–28.
- Hodge, James, Johnson, Brian, and Rimas Orentas. Cancer Vaccines and Tumor Immunity . John Wiley & Sons, Inc. 2008.
- HPV Vaccine. "The HPV Vaccine Program." HPV Vaccine. http://www.hpvvaccine.org.au/the-hpv-vaccine/why-was-the-program-introduced.aspx (Accessed May 6, 2016).
- NobelPrize.org. "Harald zur Hausen — Biographical." NobelPrize.org http://www.nobelprize.org/nobel_prizes/medicine/laureates/2008/hausen-bio.html (Accessed May 6, 2016).
- Markowitz, L., Hariri, S., Lin, C., Dunne, E., Steinau, M., McQuillan, G., and Unger, E. "Reduction in Human Papillomavirus (HPV) Prevalence Among Young Women Following HPV Vaccine Introduction in the United States, National Health and Nutrition Examination Surveys, 2003-2010." The Journal of Infectious Diseases (2013): 1-9.
- McCance, Dennis, Ed. Human Papilloma Viruses . New York, New York, 2002.
- McIntyre, Peter. "Finding the Viral Link: Harald zur Hausen." Cancer World . http://www.cancerworld.org/pdf/6737_cw7_32_37_Masterpiece%20%282%29.pdf (Accessed May 6, 2016).
- Medline Plus. "Pap Smear." National Institutes of Health. http://www.nlm.nih.gov/medlineplus/ency/article/003911.htm (Accessed May 6, 2016).
- National Cancer Institute. "Human Papilloma Virus (HPV) Vaccines." National Cancer Institute. http://www.cancer.gov/cancertopics/causes-prevention/risk/infectious-agents/hpv-vaccine-fact-sheet (Accessed May 6, 2016).
- Papaniocolaou, George Nikolaou and Herbert Federick Traut. "The diagnostic value of vaginal smears in carcinoma of the uterus." American Journal of Obstetrics and Gynecology 42 (1941): 193–206.
- Ratanasiripong, N. "A Review of Human Papillomavirus (HPV) Infection and HPV Vaccine-Related Attitudes and Sexual Behaviors among College-Aged Women in the United States." Journal of American College Health 60 (2012): 461-470.
- Shepard, Elizabeth. "George Papanicolaou: Development of the Pap Smear." Weill Cornell. http://weill.cornell.edu/archives/blog/2011/06/george-papanicolaou-development-of-the-pap-smear.html (Accessed May 6, 2016).
- Stokley, Shannon et. al. "Human Papillomavirus Vaccination Coverage Among Adolescents, 2007-2013, and Postlicensure Vaccine Safety Monitoring, 2006-2014 — United States." CDC: Morbidity and Mortality Weekly Report 63 (2014): 620–4.
- Tjalma, Wiebren. "There are two prophylactic human papillomavirus vaccine against cancer, and they are different." Journal of Clinical Oncology 33 (2015): 1–3.
- zur Hausen, Harald. "Condylomata Acuminata and Human Genital Cancer." Cancer Research 36 (1976): 794.
- zur Hausen, Harald. "Papillomaviruses in the causation of human cancers—a brief historical account." Virology 384 (2009): 260–5.
Keywords
Editor
How to cite
Publisher
Handle
Rights
Articles Rights and Graphics
Copyright Arizona Board of Regents Licensed as Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported (CC BY-NC-SA 3.0)